Latest news :

Research & Development
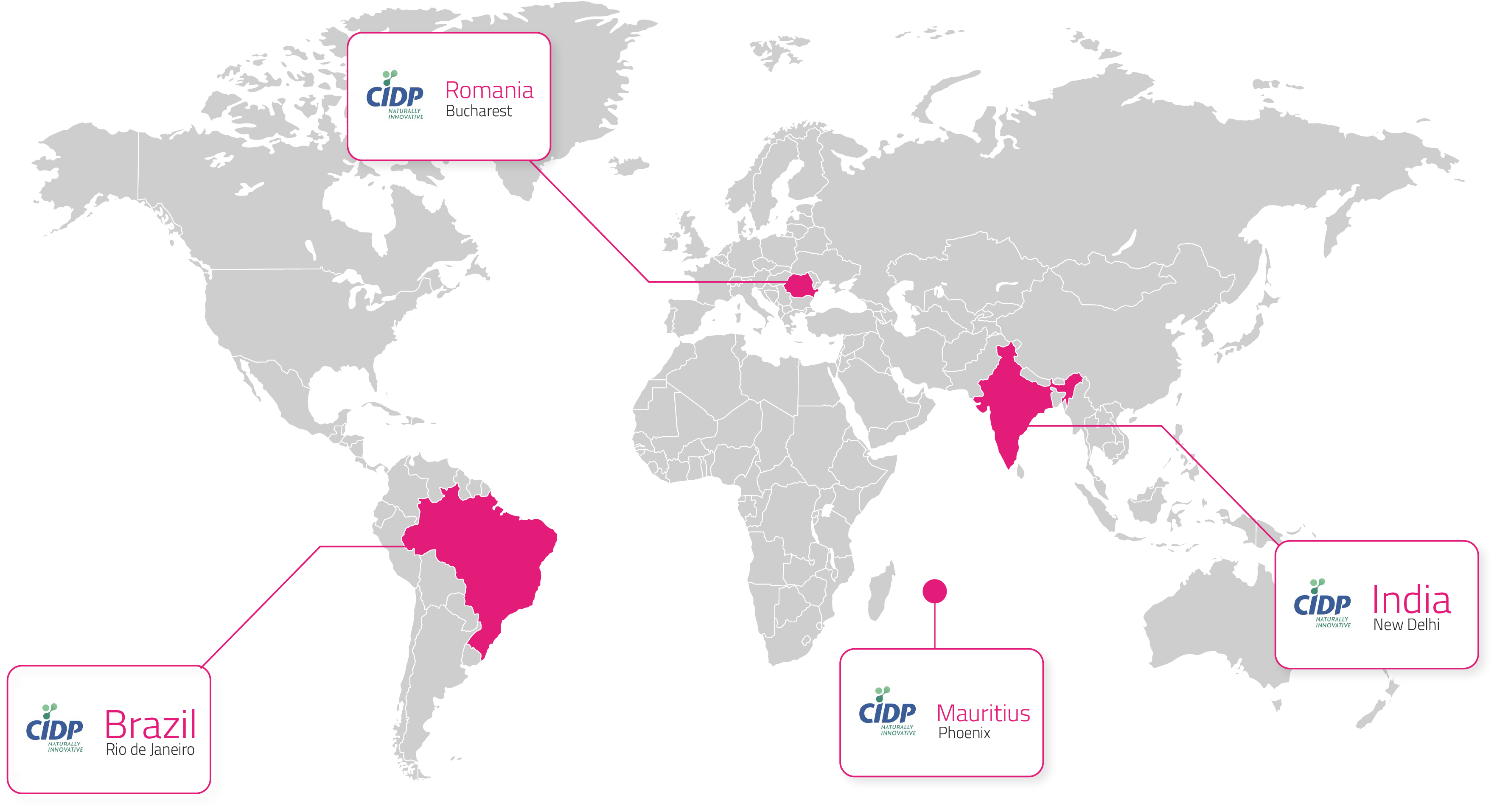
Centre International de Développement Pharmaceutique (CIDP) is a global Contract Research Organization (CRO) specializing in dermatological research. With over 20 years of expertise, we conduct high-quality clinical trials to evaluate the safety and efficacy of skincare, haircare, medical devices, and pharmaceutical products. Our studies contribute to scientific advancements and support the development of innovative solutions in the cosmetics and pharmaceutical industries. Our subsidiaries are based in Brazil, India and Romania.
/ 05 Services
/ 04 Location
Our Services





Our Executive Team

Dr Claire Blazy Jauzac
Group CEO
CIDP

Sabrina Sonea
Managing Director
CIDP

Dr Gitanjali Petkar
Dermatologist/Medical Director – Dermocosmetics
CIDP

Dr Ounisha Mungur
Medical Director – Pharmaceutical Operation
CIDP

Anissah Appadu
Head Of Projects
CIDP

Elodie Montezuma
Volunteers Recruitment Manager
CIDP
FAQs
Why Volunteer?
CIDP continuously seeks volunteers to participate in our clinical trials. By joining our studies, you contribute to scientific progress and gain access to innovative skincare and healthcare solutions under expert supervision. Our trials prioritise volunteers’ safety, adhering to strict ethical guidelines and regulatory requirement.
- Help advance research and innovation.
- Benefit from expert advice and high-quality, innovative products from well-known brands.
- Receive compensation for your valuable opinion and the constraints you experience while participating in a study.
What is a clinical trial?
A clinical trial is a research study that evaluates the safety and efficacy of new products. At CIDP, we specialize in dermatological and cosmetic clinical trials, helping beauty and wellness brands bring innovative, safe, and effective products to market while adhering to the highest ethical standards.
What types of clinical trials does CIDP conduct?
You can contact us by phone: 267 0270 or e-mail: info@theact.mu
Who can participate in CIDP clinical trials?
Our studies are open to healthy volunteers as well as individuals with specific skin concerns, depending on the trial requirements. Each study has its own specific requirements about who can and can’t participate. We also conduct trials for kids’ and babies’ skincare products, ensuring their efficacy for the youngest users.
Are CIDP clinical trials safe?
Yes. We adhere to strict ethical standards and regulations, including Good Clinical Practices (GCP). All studies are supervised by qualified medical professionals to ensure participant safety.
Will I be compensated for participating?
Yes, at the end of any study, you will receive compensation for sharing your opinion on the product by completing all required logs and detailing any constraints you encountered during your participation.
How can I sign up for a clinical trial?
Please visit our website at https://volunteers-mu.cidp-cro.com/. Click on “New to CIDP, Join Us” and fill out the questionnaire to pre-register in our database. Once this step is completed, you will be registered in our volunteer database and will be informed of ongoing studies via SMS.
How do I know if I qualify for a specific study?
Each study has specific requirements. If you’re interested, our team will schedule an appointment with one of our in-house doctors (investigators) to assess your suitability based on the study requirements. An initial pre-screening will be conducted via phone calls. Preselected persons will then visit the centre to validate their criteria as per the study design. Only persons validated by our investigators will receive the investigational product (except for consumer tests, where the technician will determine if the person meets the specific requirements)).
How long do clinical trials last?
The duration of a trial varies based on its design. Some trials may last a few weeks, while others may require longer participation. The timeline will be provided before enrolment.
What happens during a clinical trial?
Participants undergo either dermatological, ophthalmological, or paediatric assessments. They use the study product as directed and attend follow-up visits for monitoring and evaluation.
Can I stop my participation in a study after being included?
Yes, participation is voluntary, and you can stop your participation in the study at any time without any consequences.
How will my personal information be handled?
All personal data is kept confidential and used strictly for research purposes, following data protection regulations. CIDP has been awarded a Platinum Medal by CyberVadis for strong cybersecurity measures. You can contact our Data Protection Officer (DPO) at any time for access to or deletion of your data. Before being registered in our database, each subject will need to pre-fill data protection forms to give their consent (either hard copies or online). Their data will be kept for 10 years. A subject can request at any time to delete or obtain a copy of their data by contacting our DPO at dpo@cidp-cro.com.
Subscribe
to our Newsletter!

Together, improving people’s health for a better life
Our expertise
About us
An IBL Group Company - © 2025 All Rights Reserved - Privacy notice - Website created by Bulle studio